EGFR Knockout Cell Line-HeLa
Cat.No. : CSC-RT1811
Host Cell: HeLa Target Gene: EGFR
Size: 1x10^6 cells/vial, 1mL Validation: Sequencing
Cat.No. : CSC-RT1811
Host Cell: HeLa Target Gene: EGFR
Size: 1x10^6 cells/vial, 1mL Validation: Sequencing
| Cat. No. | CSC-RT1811 |
| Cell Line Information | HeLa -EGFR(-/-) is a stable cell line with a homozygous knockout of human EGFR using CRISPR/Cas9. |
| Target Gene | EGFR |
| Host Cell | HeLa |
| Shipping | 1 vial of knockout cell line |
| Storage | Liquid nitrogen |
| Species | Homo sapiens (Human) |
| Gene Symbol | EGFR |
| Gene ID | 1956 |
| Revival | Rapidly thaw cells in a 37°C water bath. Transfer contents into a tube containing pre-warmed media. Centrifuge cells and seed into a 25 cm2 flask containing pre-warmed media. |
| Media Type | Cells were cultured in DMEM supplemented with 10% fetal bovine serum. |
| Growth Properties | Cells are cultured as a monolayer at 37°C in a humidified atmosphere with 5% CO2. Split at 80-90% confluence, approximately 1:4-1:6. |
| Freeze Medium | Complete medium supplemented with 10% (v/v) DMSO |
| Mycoplasma | Negative |
| Format | One frozen vial containing millions of cells |
| Storage | Liquid nitrogen |
| Safety Considerations |
The following safety precautions should be observed. 1. Use pipette aids to prevent ingestion and keep aerosols down to a minimum. 2. No eating, drinking or smoking while handling the stable line. 3. Wash hands after handling the stable line and before leaving the lab. 4. Decontaminate work surface with disinfectant or 70% ethanol before and after working with stable cells. 5. All waste should be considered hazardous. 6. Dispose of all liquid waste after each experiment and treat with bleach. |
| Ship | Dry ice |
STING (stimulator of interferon genes) mediates protective cellular responses to microbial infection and tissue damage, but its aberrant activation leads to autoinflammatory diseases. Upon ligand stimulation, the endoplasmic reticulum (ER) protein STING translocates to endosomes to induce interferon production, whereas an alternative trafficking pathway delivers it directly to autophagosomes. Here, researchers show that phosphorylation of specific tyrosine residues in STING by the epidermal growth factor receptor (EGFR) is required to direct STING to endosomes, where it interacts with the downstream effector IRF3. In the absence of EGFR-mediated phosphorylation, STING rapidly translocates to autophagosomes, and IRF3 activation, interferon production, and antiviral activity are impaired in cell culture and mice, whereas autophagic activity is enhanced.
Here, researchers show that STING signaling requires the action of EGFR to activate IRF3, but not NF-κB (Figure 1A-D). STING activation of IRF3 requires the interaction of both proteins as well as TBK1 (IRF3 kinase). After cGAMP stimulation, IRF3 co-immunoprecipitated with STING, but this interaction did not occur after gefitinib treatment (Figure 1D) or in EGFR knockout cells (Figure 1E). Confocal microscopy also demonstrated STING-IRF3 interaction (Figure 1F). After activation, IRF3 may leave STING and move to the nucleus (Figure 1H). The above analysis concluded that STING recruitment to IRF3 and IRF3 activation occur in late endosomes and that they require EGFR kinase activity. However, in the absence of EGFR activity, STING Ser366 phosphorylation was not affected. The kinetics of Ser366 phosphorylation were very similar in WT Hela cells, EGFR knockout Hela cells, and gefitinib-treated Hela cells (Figure 1G), indicating that EGFR does not act by promoting Ser366 phosphorylation.
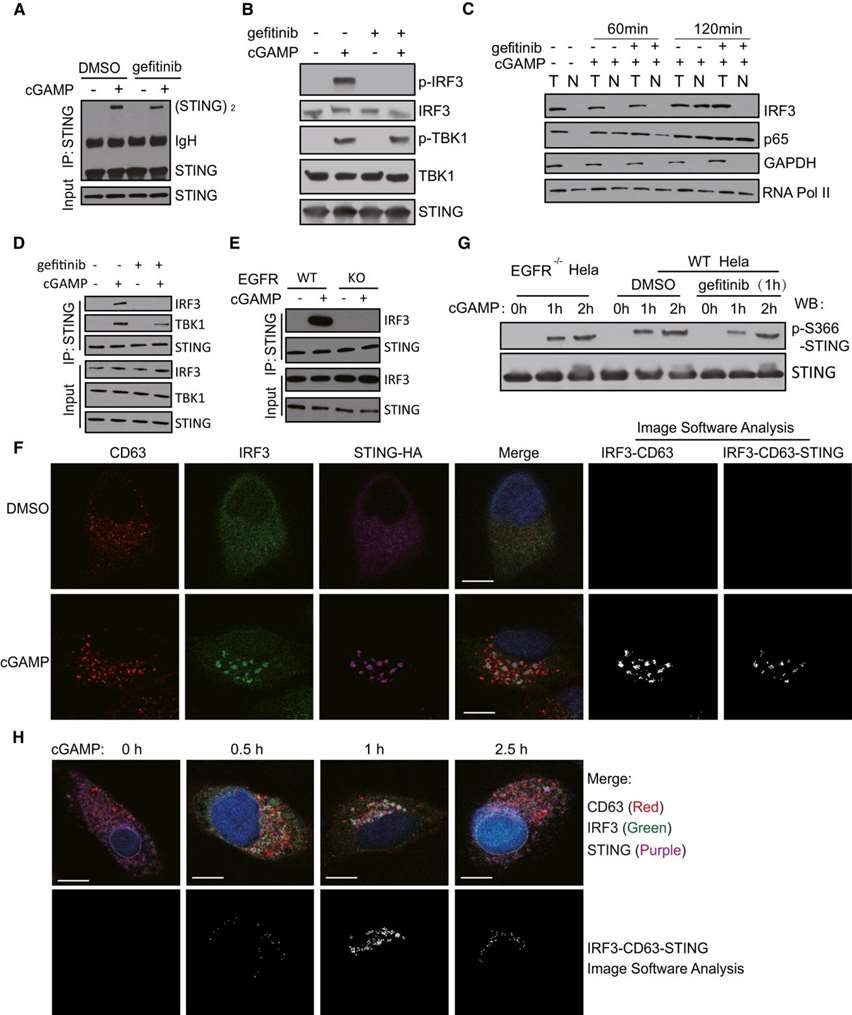 Figure 1. EGFR kinase activity is required for IRF3 activation. (Wang C, et al., 2020)
Figure 1. EGFR kinase activity is required for IRF3 activation. (Wang C, et al., 2020)

Our promise to you:
Guaranteed product quality, expert customer support.
 24x7 CUSTOMER SERVICE
24x7 CUSTOMER SERVICE
 CONTACT US TO ORDER
CONTACT US TO ORDER