Human LDLR Knockout Cell Line-HEK293T
Cat.No. : CSC-RT2645
Host Cell: HEK293T Target Gene: LDLR
Size: 2x10^6 cells/vial, 1mL Validation: Sequencing
Cat.No. : CSC-RT2645
Host Cell: HEK293T Target Gene: LDLR
Size: 2x10^6 cells/vial, 1mL Validation: Sequencing
| Cat. No. | CSC-RT2645 |
| Cell Line Information | This cell line is a stable cell line with a homozygous knockout of human LDLR using CRISPR/Cas9. |
| Target Gene | LDLR |
| Gene ID | 3949 |
| Genotype | LDLR (-/-) |
| Host Cell | HEK293T |
| Cell Type | Epithelial |
| Size | 2x10^6 cells/vial, 1mL |
| Sequencing Result | Homozygous: 44 bp deletion in exon |
| Species | Homo sapiens (Human) |
| Revival | Rapidly thaw cells in a 37°C water bath. Transfer contents into a tube containing pre-warmed media. Centrifuge cells and seed into a 25 cm2 flask containing pre-warmed media. |
| Media Type | Cells were cultured in DMEM supplemented with 10% fetal bovine serum. |
| Growth Properties | Cells are cultured as a monolayer at 37°C in a humidified atmosphere with 5% CO2. Split at 80-90% confluence, approximately 1:3-1:6. |
| Freeze Medium | Complete medium supplemented with 10% (v/v) DMSO |
| Mycoplasma | Negative |
| Format | One frozen vial containing millions of cells |
| Storage | Liquid nitrogen |
| Safety Considerations |
The following safety precautions should be observed. 1. Use pipette aids to prevent ingestion and keep aerosols down to a minimum. 2. No eating, drinking or smoking while handling the stable line. 3. Wash hands after handling the stable line and before leaving the lab. 4. Decontaminate work surface with disinfectant or 70% ethanol before and after working with stable cells. 5. All waste should be considered hazardous. 6. Dispose of all liquid waste after each experiment and treat with bleach. |
| Ship | Dry ice |
Lentiviral vectors (LVs) are reliable vehicles for gene therapy because they can efficiently integrate transgenes into the host cell genome. However, LVs with long or complex expression cassettes are often produced at low titers and have reduced gene transfer capacity, creating barriers to clinical and commercial applications. Improvements in packaging cell lines and methods may enable the production of complex vectors with higher titers and infectivity and could improve the production of many different LVs. In this study, researchers identified two host restriction factors that hinder LV production in HEK293T packaging cells, 2′-5′-oligoadenylate synthetase 1 (OAS1) and low-density lipoprotein receptor (LDLR). Knockout of these two genes individually resulted in an approximately 2-fold increase in viral titers. The researchers created a monoclonal cell line, CRISPRed HEK293T to Disrupt Antiviral Response (CHEDAR), by sequentially knocking out OAS1, LDLR, and PKR, a factor previously found to hinder LV titers. Packaging in CHEDAR resulted in an approximately 7-fold increase in physical particles, full-length vector RNA, and vector titer.
Because homozygous deletion of ATR results in chromosome breakage and failure to proliferate in culture, researchers selected ATR KD clones with 63% indel and 63% KO rates, which showed significantly reduced protein expression (Figure 1A). Interferon α and β receptor subunit 1 (IFNAR1), OAS1, and LDLR were successfully knocked out, as shown in Figures 1A and 1B. Unstimulated CD34+ hematopoietic stem and progenitor cells (HSPCs), which are known to have no or low expression of LDLR28, were used as negative controls when measuring LDLR expression in LDLR knockout cells by flow cytometry (Figure 1B). Targeted CRISPR-Cas9 screening showed that individual disruption of IFNAR1, OAS1, ATR, and LDLR genes each increased titer by approximately 2-fold (Figure 1C). PKR knockout HEK293T cells had a 4.1±1.1-fold increase in titer. IFNAR1 knockout HEK293T cells had a 2.4±0.7-fold increase in titer. OAS1 knockout HEK293T cells had a 2.3±0.8-fold increase in titer. ATR knockdown cells had a 2.2±0.8-fold increase in titer. LDLR knockout HEK293T cells had a 2.8±0.9-fold increase in titer.
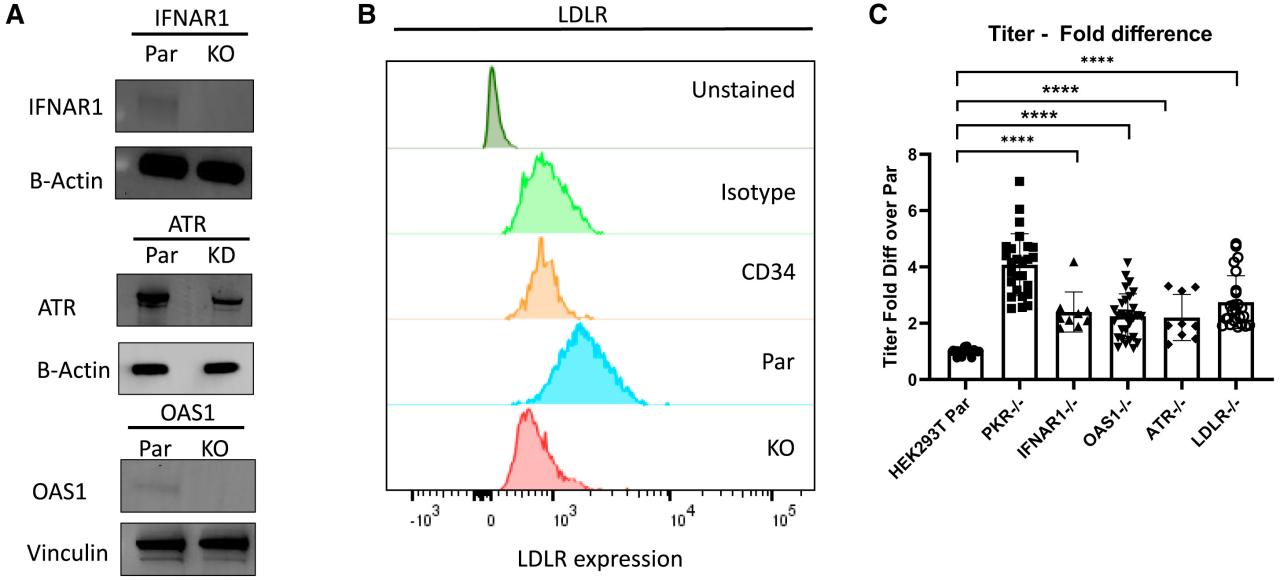 Figure 1. Knocking out IFNAR1, ATR, OAS1, and LDLR in 293T cells increased titers. (Han J, et al., 2021)
Figure 1. Knocking out IFNAR1, ATR, OAS1, and LDLR in 293T cells increased titers. (Han J, et al., 2021)

Our promise to you:
Guaranteed product quality, expert customer support.
 24x7 CUSTOMER SERVICE
24x7 CUSTOMER SERVICE
 CONTACT US TO ORDER
CONTACT US TO ORDER