Human FGFR2 Knockout Cell Line-HEK293T
Cat.No. : CSC-RT0663
Host Cell: HEK293T Target Gene: FGFR2
Size: 1x10^6 cells/vial, 1mL Validation: Sequencing
Cat.No. : CSC-RT0663
Host Cell: HEK293T Target Gene: FGFR2
Size: 1x10^6 cells/vial, 1mL Validation: Sequencing
| Cat. No. | CSC-RT0663 |
| Cell Line Information | A stable cell line with a homozygous knockout of human FGFR2 using CRISPR/Cas9. |
| Target Gene | FGFR2 |
| Host Cell | HEK293T |
| Host Cell Species | Homo sapiens (Human) |
| Shipping | 10^6 cells/tube |
| Storage | Liquid nitrogen |
| Gene ID | 2263 |
| Revival | Rapidly thaw cells in a 37°C water bath. Transfer contents into a tube containing pre-warmed media. Centrifuge cells and seed into a 25 cm2 flask containing pre-warmed media. |
| Media Type | Cells were cultured in DMEM supplemented with 10% fetal bovine serum. |
| Growth Properties | Cells are cultured as a monolayer at 37°C in a humidified atmosphere with 5% CO2. Split at 80-90% confluence, approximately 1:3-1:6. |
| Freeze Medium | Complete medium supplemented with 10% (v/v) DMSO |
| Mycoplasma | Negative |
| Format | One frozen vial containing millions of cells |
| Storage | Liquid nitrogen |
| Safety Considerations |
The following safety precautions should be observed. 1. Use pipette aids to prevent ingestion and keep aerosols down to a minimum. 2. No eating, drinking or smoking while handling the stable line. 3. Wash hands after handling the stable line and before leaving the lab. 4. Decontaminate work surface with disinfectant or 70% ethanol before and after working with stable cells. 5. All waste should be considered hazardous. 6. Dispose of all liquid waste after each experiment and treat with bleach. |
| Ship | Dry ice |
NF2 is a tumor suppressor gene that is frequently mutated in malignant pleural mesothelioma (MPM). Here, we found that cell growth, clonogenic activity, migration activity, and invasion activity of the NF2-knockout human mesothelial cell line MeT-5A (NF2-KO) were significantly increased compared with NF2-WT cell clones. Complementary DNA microarray analysis clearly revealed differences in the global gene expression profiles between NF2-WT and NF2-KO cell clones. Quantitative PCR analysis and Western blot analysis showed that upregulation of fibroblast growth factor receptor 2 (FGFR2) was concurrent with increased phosphorylation levels of JNK, c-Jun, and retinoblastoma (Rb) in NF2-KO cell clones. These increases were all abolished by exogenous NF2 expression in NF2-KO clones. Furthermore, disruption of FGFR2 in NF2-KO cell clones inhibited cell proliferation as well as the phosphorylation levels of JNK, c-Jun, and Rb. These findings suggest that NF2 deficiency may play a role in tumorigenesis of human mesothelial cells by mediating FGFR2 expression. FGFR2 will be a candidate molecule for the development of therapeutic and diagnostic strategies for NF2-deficient MPM.
MTT assays showed that the cell growth rate in NF2 and FGFR2 double knockout cell clones (NF2/FGFR2-DKO) was significantly reduced compared with NF2-KO clones (Figure 1A). In contrast, there was no significant change in the growth rate between FGFR2 knockout cells (FGFR2-KO) and parental cells (Figure 1B). In addition, disruption of FGFR2 in NF2-KO cells suppressed the NF2 knockout-induced migration and wound healing activities of NF2/FGFR2-DKO cells (Figures 1C,D). In addition, Western blot analysis showed that the phosphorylation levels of JNK and c-Jun were downregulated in NF2/FGFR2-DKO clones (Figure 1E). The researchers also found that the protein level of CDK2 and the phosphorylation level of Rb were reduced in NF2/FGFR2-DKO clones (Figure 1E). These results suggest that FGFR2 may play an important role in the proliferation of NF2-mutated mesothelioma cells.
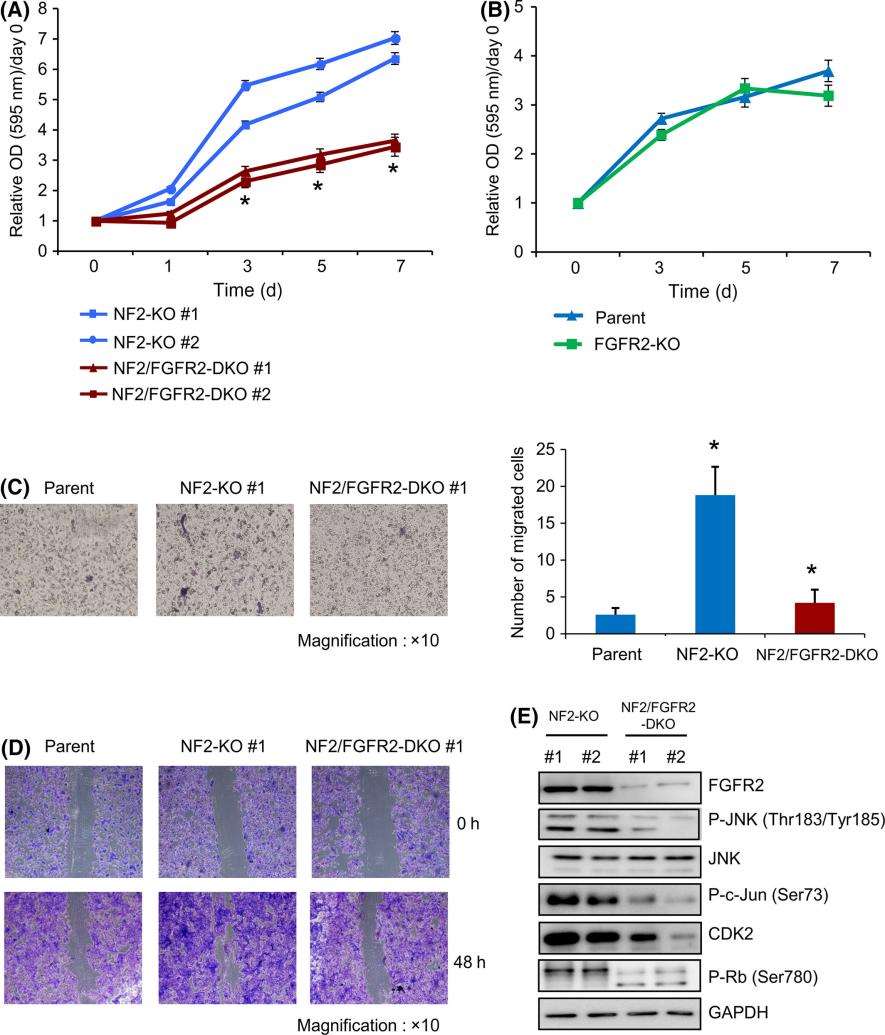 Figure 1. Knockout of FGFR2 gene retards cell proliferation in the absence of NF2 gene. (Wahiduzzaman, Md, et al. 2019)
Figure 1. Knockout of FGFR2 gene retards cell proliferation in the absence of NF2 gene. (Wahiduzzaman, Md, et al. 2019)

Our promise to you:
Guaranteed product quality, expert customer support.
 24x7 CUSTOMER SERVICE
24x7 CUSTOMER SERVICE
 CONTACT US TO ORDER
CONTACT US TO ORDER